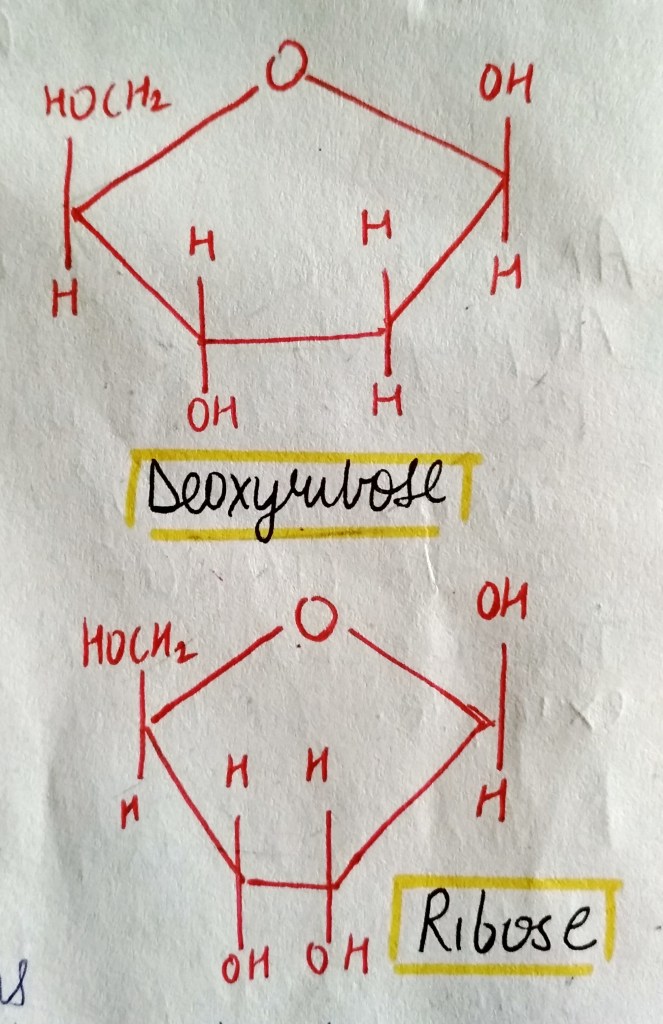
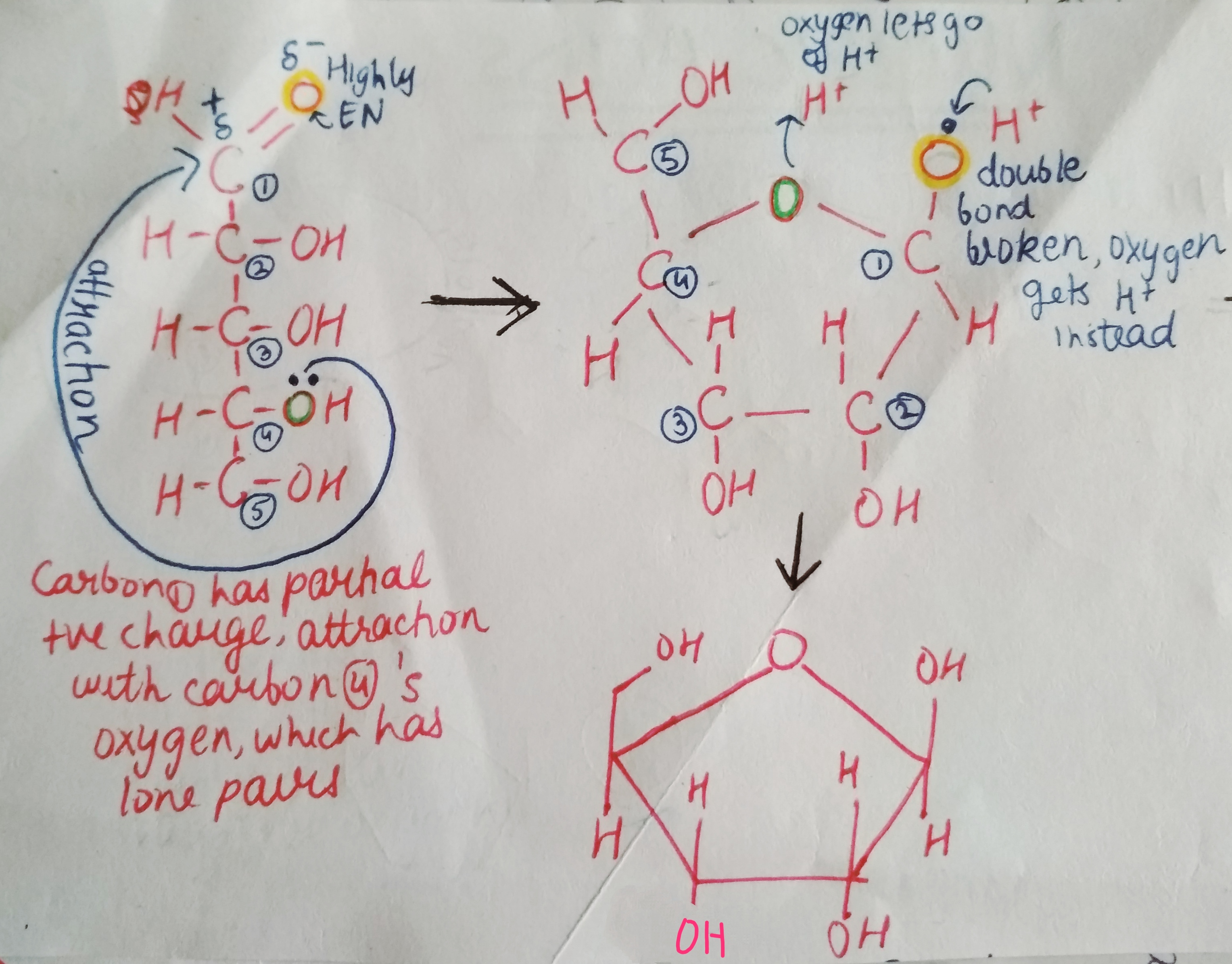
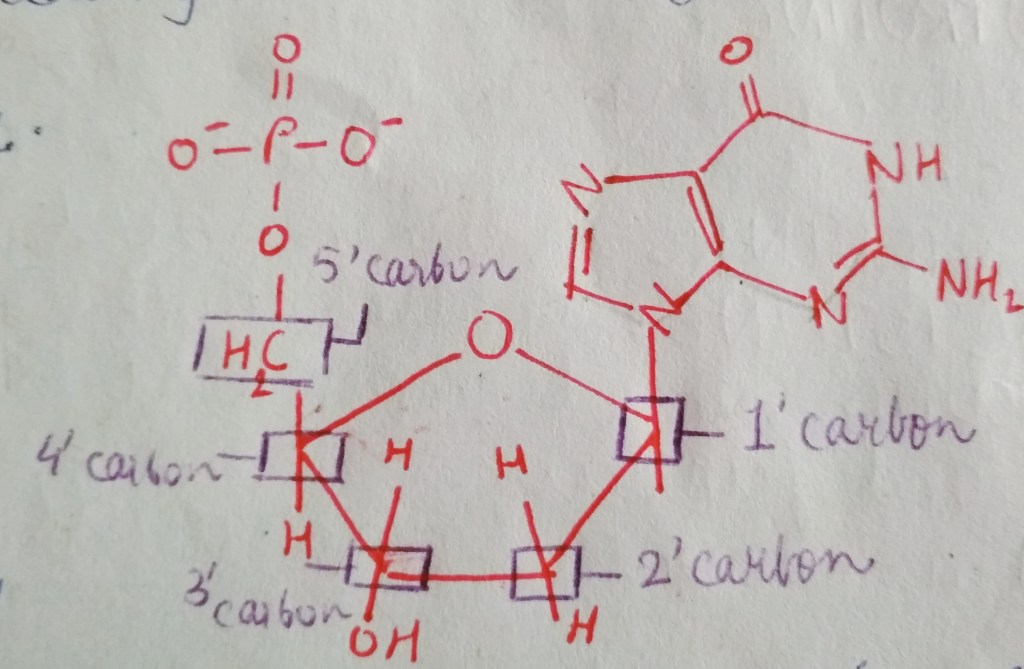
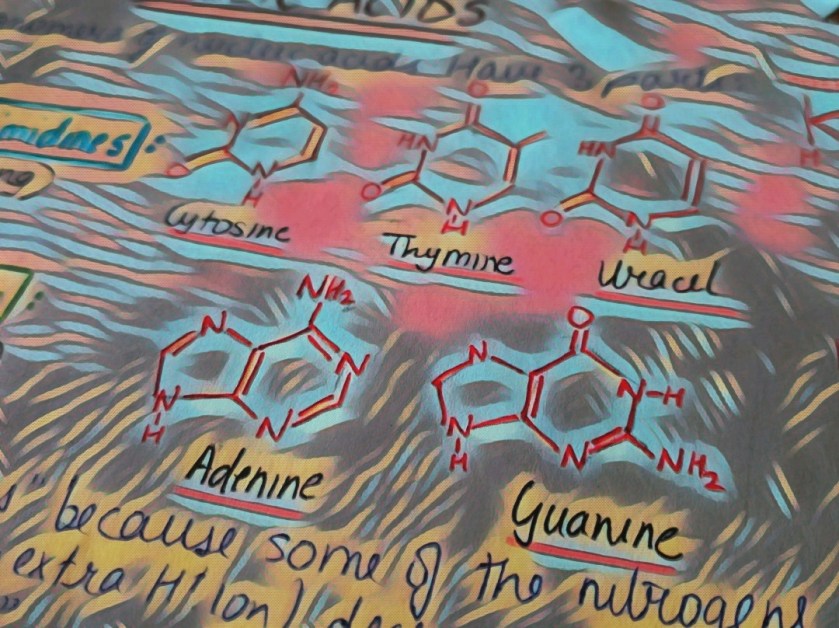
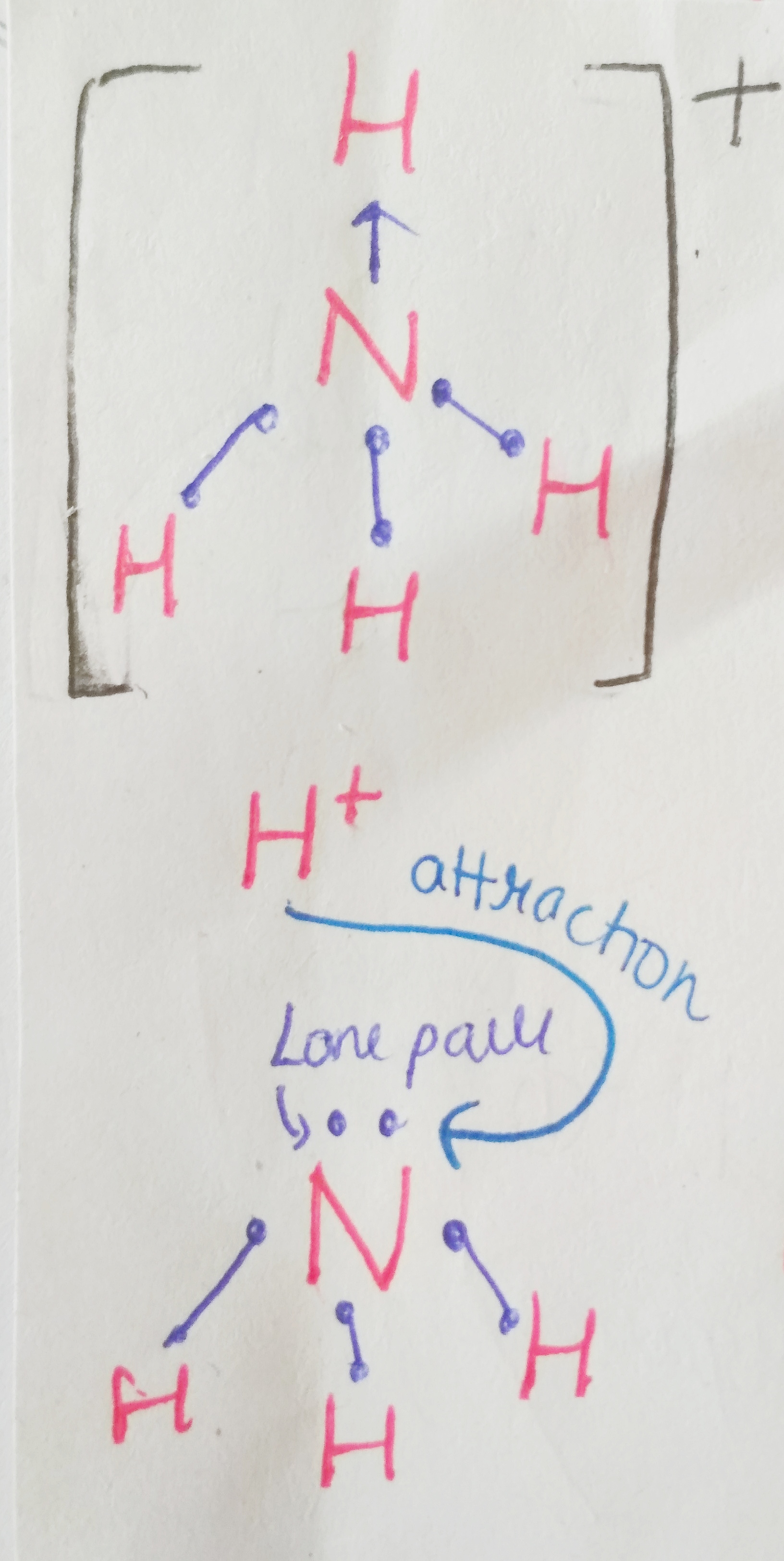
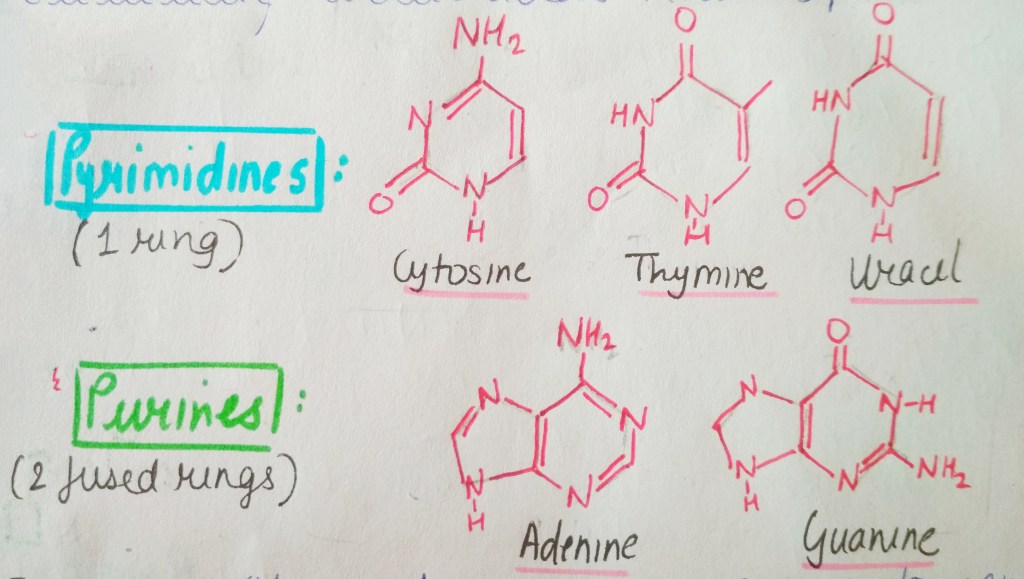
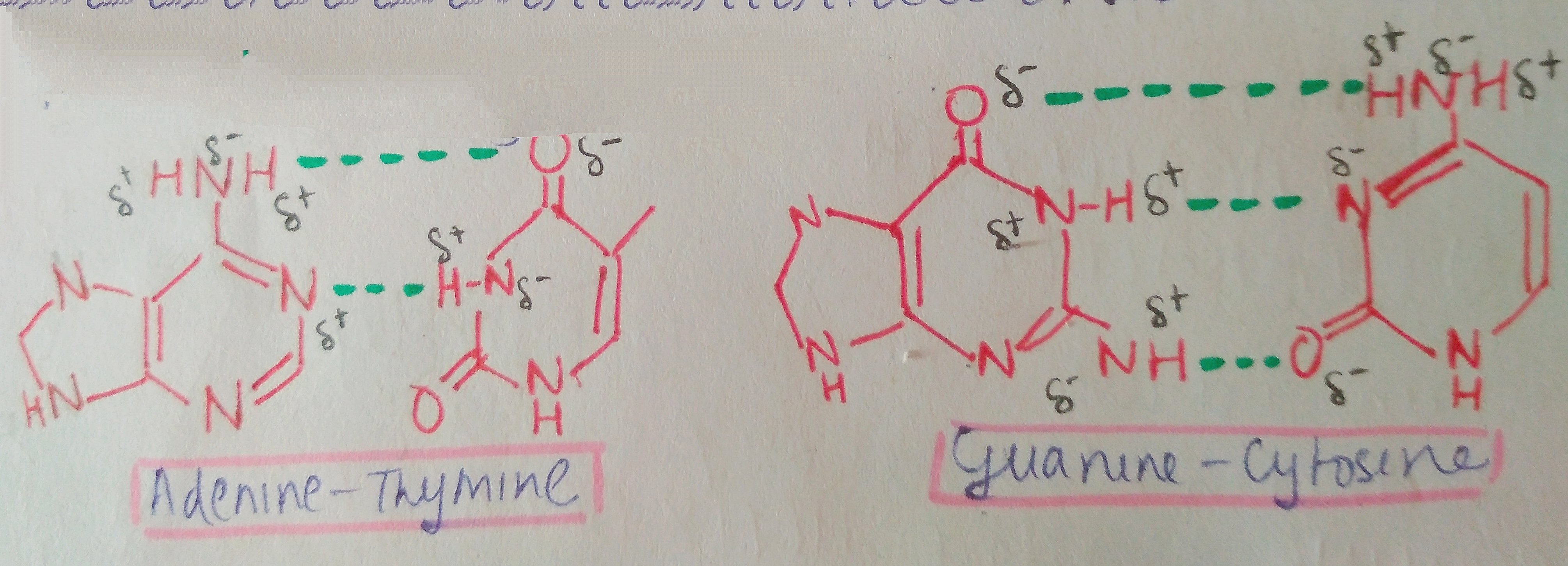
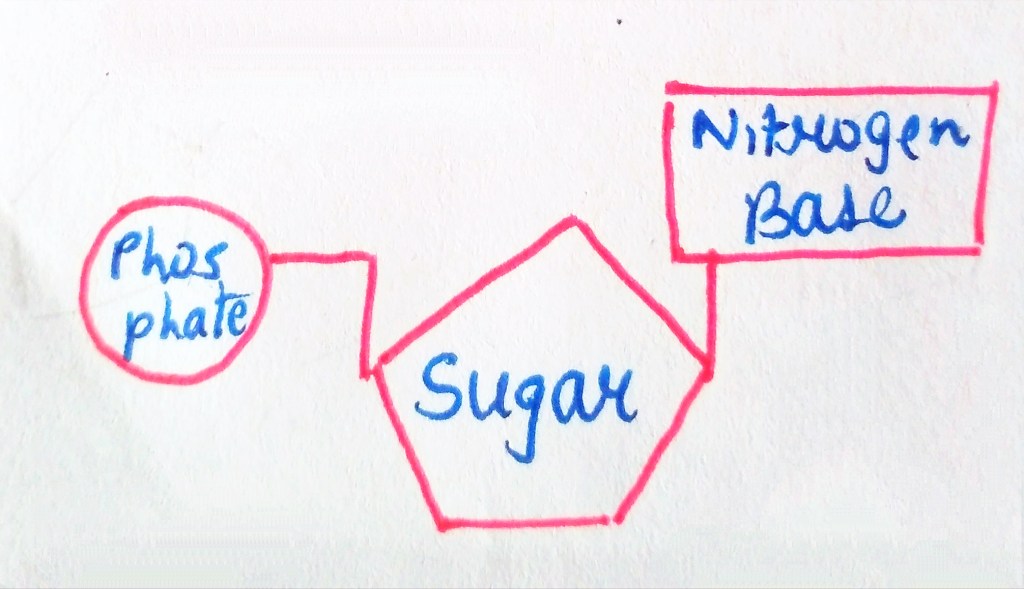
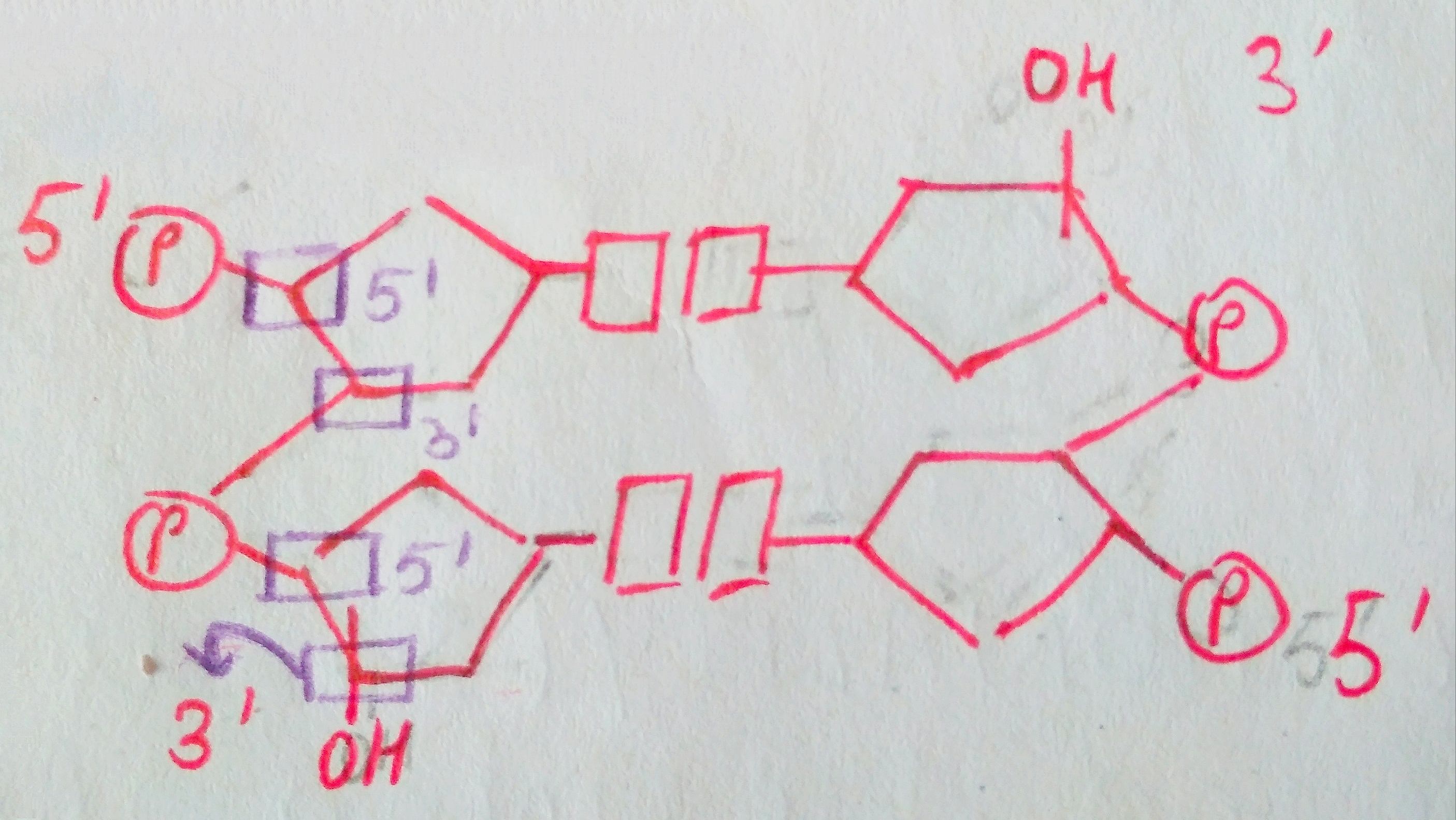
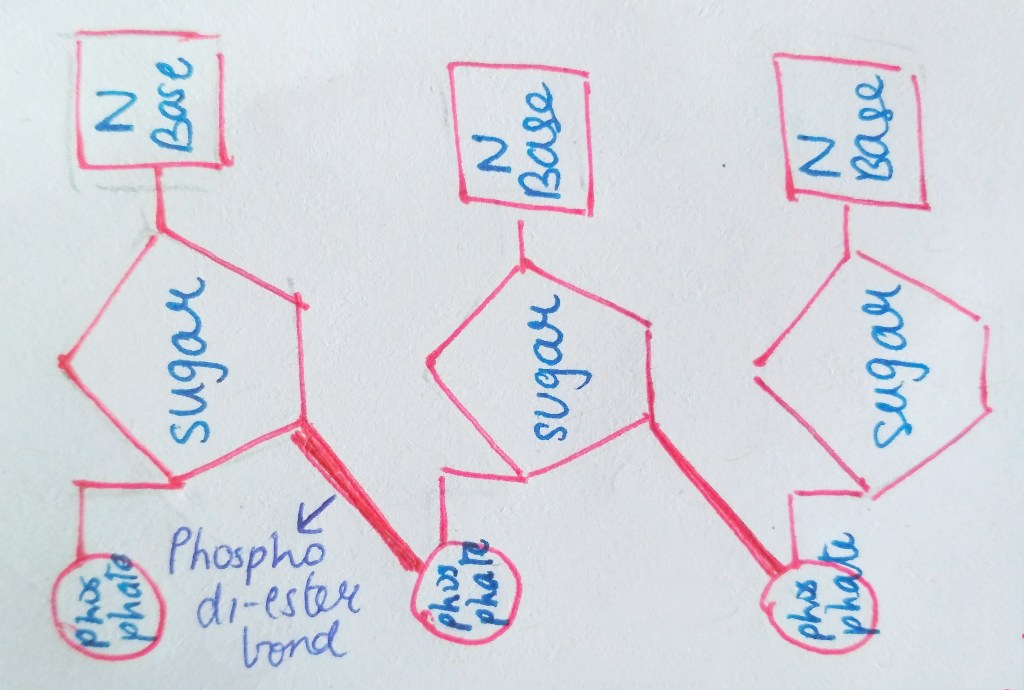
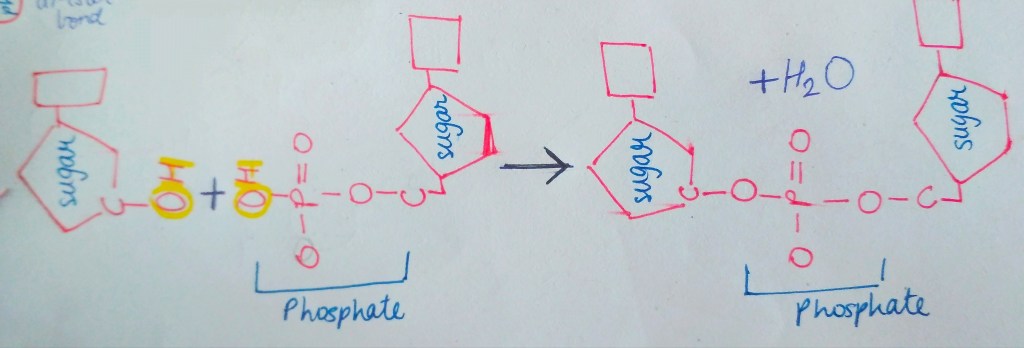
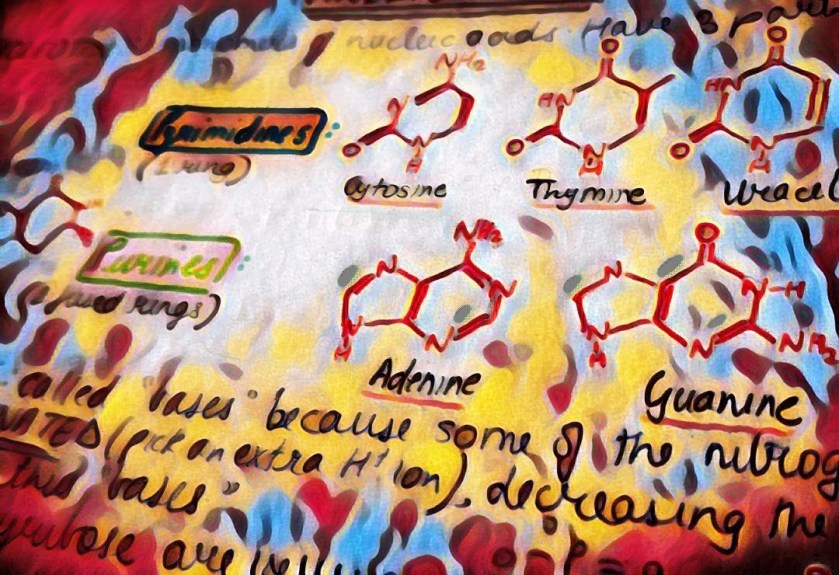
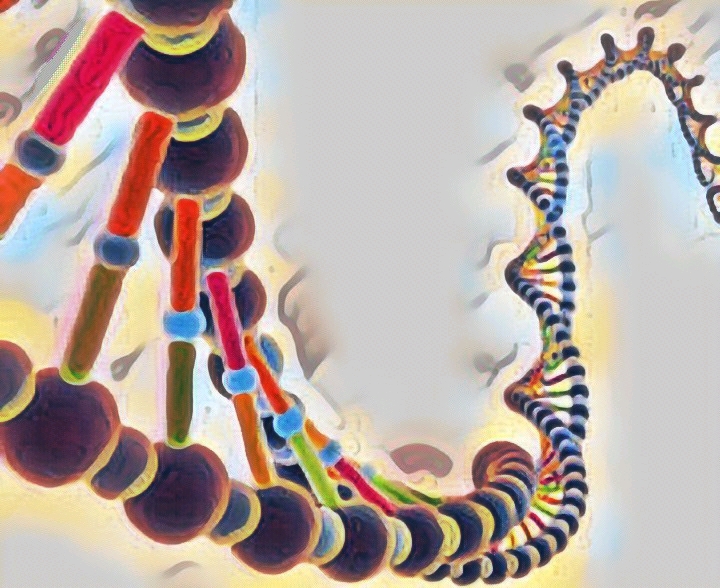
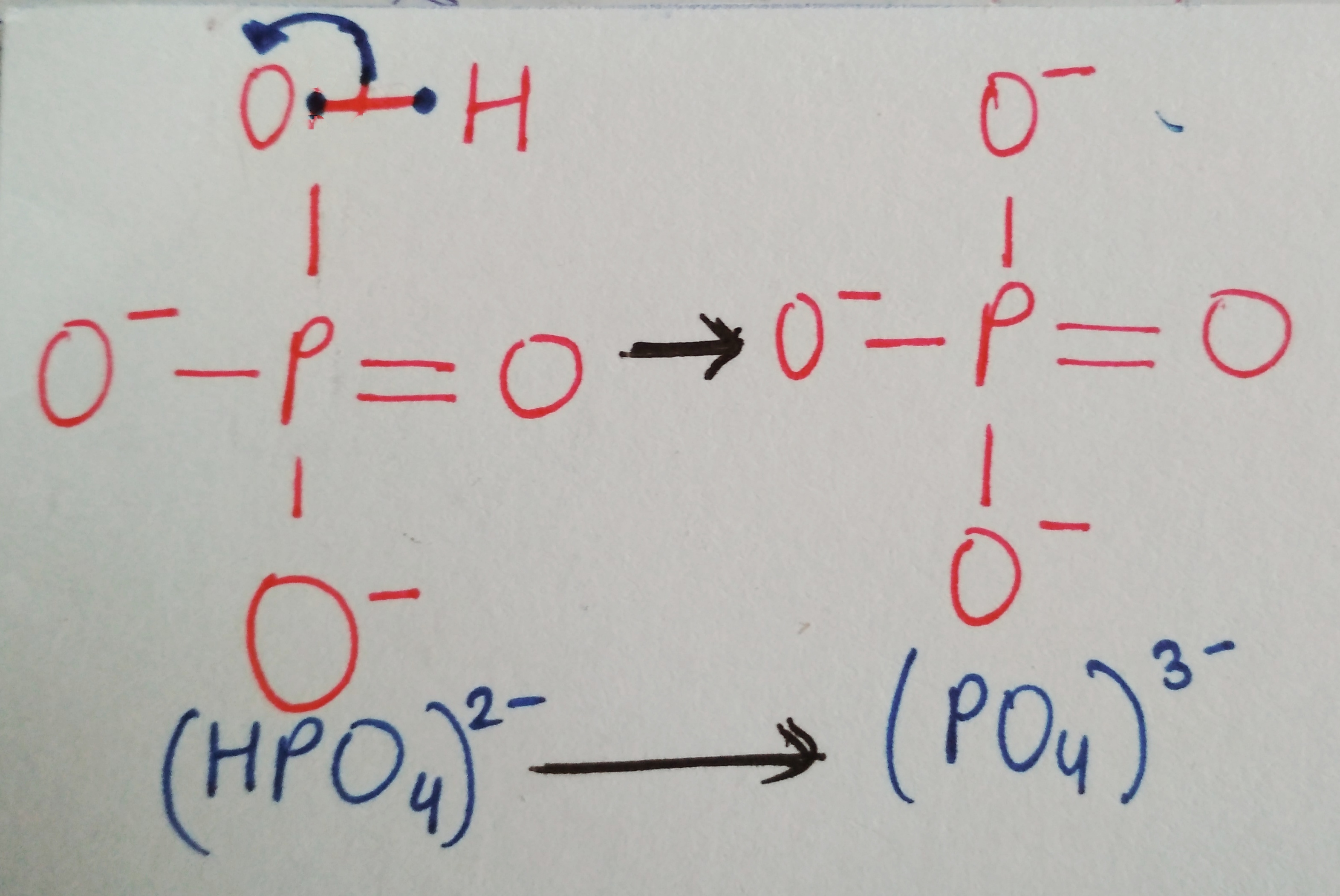
We are not getting philosophical today. In the world of biology, PHOSPHATE happens to be a crucial ion, considering that it plays such an important role in providing our cells with energy. it is also a part of nucleic acids, forming the “Sugar-Phosphate Backbone”. it is because of the phosphate group that DNA and RNA are termed as “Acids”, despite the presence of Nitrogenous “Bases” in them. a typical DNA and RNA has Hydrogenphosphate (HPO4)2-, but in aqueous medium, the H+ dissociates immediately, making it acidic in nature.
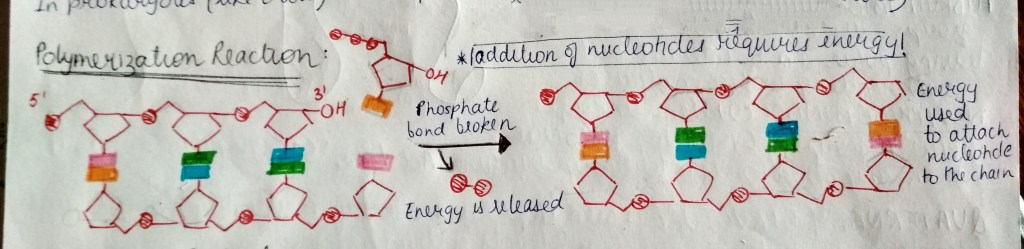
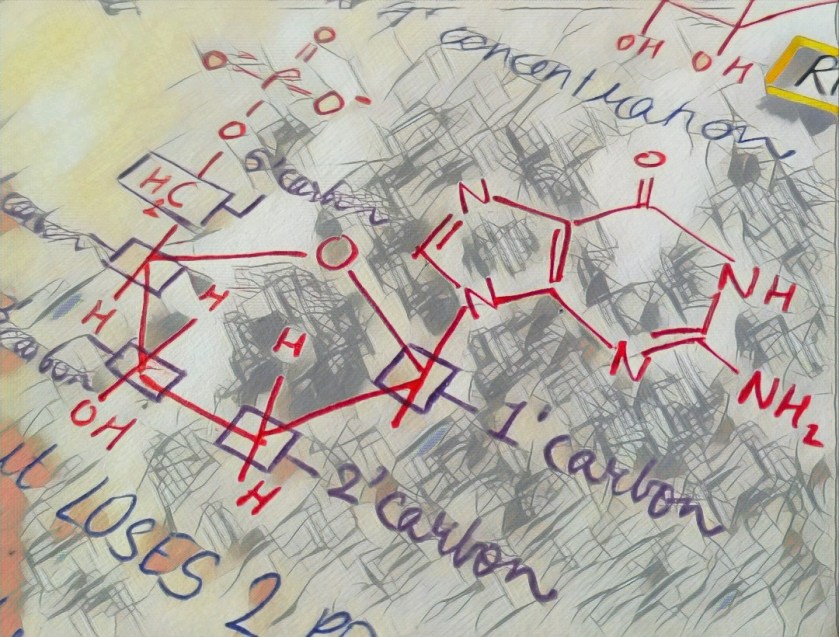
in a cell, a nucleotide is actually present with 3 phosphate groups attached to it. when a particular nucleotide is about to become a part of a growing DNA/RNA, it loses 2 of its phosphate groups, releasing energy. this energy is used in addition of nucleotides to the DNA/RNA chain. (image of nucleotide attaching and energy)